A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) more than one of these
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct structure of 2-methyl-1,3-butadiene?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of the above.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about conformations of methylcyclohexane is true? 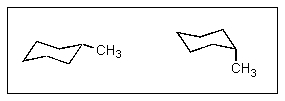
A) The energy barrier to interconvert these is too high to be achieved at room temperature.
B) The two forms are in equilibrium and are present in equal amounts at room temperature.
C) The two forms are not in equilibrium but are present in equal amounts at room temperature.
D) The two forms are in equilibrium but are not present in equal amounts at room temperature.
E) The two forms are not in equilibrium and are not present in equal amounts at room temperature.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cyclohexanes exhibit a higher_______than their straight-chain analogs.(Choose the correct answer)
A) boiling point
B) melting point
C) density
D) All of these are correct.
E) Two of these are correct.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following cyclic alkanes has the greatest tendency to have a planar ring?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) None of the above are planar.
G) C) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What is the correct name for the following molecule? 
A) 1-chloro-2-methyl-4-propylcyclopentane
B) 2-chloro-1-methyl-4-propylcyclopentane
C) 1-chloro-5-methyl-3-propylcyclopentane
D) 5-methyl-1-chloro-3-propylcylopentane
E) 1-chloro-3-propyl-5-methylcyclopentane
G) C) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following could be classified as a terpene?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly shows the Newman projection along a C-C bond in cyclohexane? (the squiggles indicate where the rest of the ring is attached) 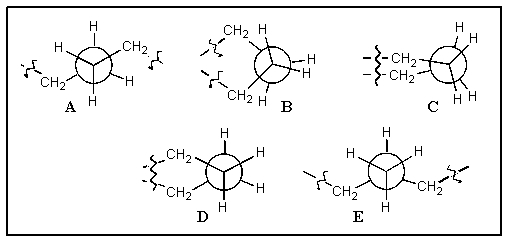
A) A
B) B
C) C
D) D
E) E
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Steroids frequently function as _____________,which are regulators of biochemical activity.
A) proteins
B) nucleic acids
C) hormones
D) fatty acids
E) triglycerides
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most stable conformation of cis-1,3-dimethylcyclohexane has how many hydrogen atoms in axial positions?
A) 4
B) 5
C) 6
D) 8
E) None of the above.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the name of the following? 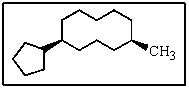
A) 5-cyclopentyl-1-methylcyclononane
B) cis-1-cyclopentyl-5-methylcyclodecane
C) cis-5-methyl-1-cyclopentylcyclododecane
D) trans-cyclopentyl-5-methylcyclodecane
E) (5-methylcyclodecyl) cyclopentane
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Terpenes can be considered to be built up from what units?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) All of the above
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Which of the following compounds has the lowest heat of combustion per CH2 group?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) All have equal Hcombustion.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest heat of combustion per CH2 group?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) All have equal Hcombustion.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the most stable conformation of trans-1-fluoro-4-methylcyclohexane?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ring systems belongs to the class of compounds called steroids?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following isomers would you expect to have the lowest heat of combustion? (i.e.,be most stable)
A) cis-1,2-dimethylcyclohexane
B) trans-1,3-dimethylcyclohexane
C) cis-1,4-dimethylcyclohexane
D) cis-1,3-dimethylcyclohexane
E) All should have the same heat of combustion.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures represent cis-1,4-dimethylcyclohexane? 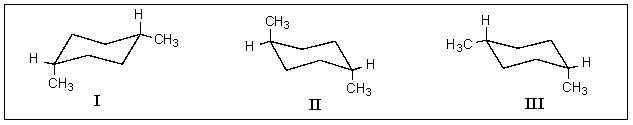
A) I & II
B) I & III
C) II & III
D) All of the above.
E) None of the above.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the proper name of the following: 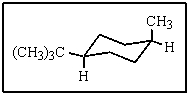
A) cis-1-tert-butyl-4-methylcyclohexane
B) trans-1-tert-butyl-4-methylcyclohexane
C) axial,equatorial-1-tert-butyl-4-methylcyclohexane
D) cis-1-isopropyl-4-methylcyclohexane
E) trans-1-isopropyl-4-methylcyclohexane
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct IUPAC name for the following molecule: 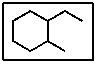
A) 1-ethyl-2-methylhexane
B) 2-ethyl-1-methylcycloheptane
C) 4-ethyl-5-methylcyclohexane
D) 1-ethyl-2-methylcyclohexane
E) 1-methyloctane
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 30
Related Exams