A) the same type of hybridization on the carbon atom
B) the same geometry around the carbon atom
C) the same number of hydrogen atoms bonded to the carbon atom
D) both carbon atoms are involved in a π bond
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many nonbonding electron pairs are in the structure shown below? 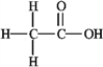
A) 2
B) 4
C) 6
D) 8
E) none of these
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw a picture showing the orbitals involved in the π-bonds of cyclopenta-1,3-diene, a commonly encountered reagent in organic synthesis. 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3 -Refer to instructions. Which of the following correctly describes the structure of these compounds?
A) All carbon atoms are sp3 hybridized.
B) All of the bonds are sigma bonds.
C) Each oxygen atom has two nonbonding pairs of electrons.
D) The bond angle around each oxygen atom is ideally about 109.5°.
E) All of these
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Covalent bonding
A) involves a transfer of electrons from one atom to another.
B) occurs when atoms share all their valence electrons.
C) occurs when unpaired valence electrons are shared between atoms.
D) occurs when nonvalence electrons are shared between atoms.
E) none of these
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Determine the hybridization for the indicated atoms in each structure below. 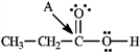
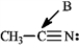 -Refer to instructions. The hybridization of carbon atom B is _____.
-Refer to instructions. The hybridization of carbon atom B is _____.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3 -Which of the following statements is not true according to molecular orbital (MO) theory?
A) Antibonding orbitals are higher in energy than the corresponding bonding orbital.
B) The head-on overlap of an s and a p atomic orbital can produce a σ molecular orbital.
C) A π molecular orbital forms only from the combination of p atomic orbital wave functions.
D) The subtractive combination of atomic orbital wave functions produces a bonding molecular orbital.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3 -What is the expected hybridization around the sulfur atom in diethyl sulfide? CH3CH2⎯S⎯CH2CH3
A) sp
B) sp2
C) sp3
D) The sulfur atom is not hybridized.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are there in the valence shell of the carbon atom of a methyl anion, CH3−?
A) 5
B) 6
C) 7
D) 8
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 29 of 29
Related Exams