A) Isobutyl bromide
B)
C) Neobutyl bromide
D)
E) Isopropyl methyl bromide
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the greatest van der Waal's interactions between its molecules?
A) ![]()
B)
C) ![]()
D)
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Draw all possible constitutional isomers for C2H6O and give a common name for each isomer.
Correct Answer

verified
Correct Answer
verified
Essay
Draw the most stable conformer of trans-1-tert-butyl-3-methylcyclohexane.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the most soluble H2CO?
A) CH3OCH3
B) CH3CH2OH
C) CH3CH2Cl
D) CH3CH2CH3
E) CH3CHO
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the structure for sec-butylcyclopentane.
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Newman projection for the least stable conformer of 3,3-dimethylhexane viewed along the C3-C4 bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound?
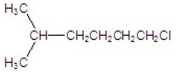
A) 2-methylheptane
B) 1-chloro-5-methylhexane
C) 6-chloro-2-methylhexane
D) 1,1-dimethyl-5-chloropentane
E) 5-methyl-1-chlorohexane
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Explain why trimethylamine, , has a considerably lower boiling point than propylamine , even though both compounds have the same molecular formula.
Correct Answer

verified
Propylamine can form hydrogen ...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw the most stable conformer of trans-1-isopropyl-2-methylcyclohexane.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The eclipsed and staggered forms of ethane differ in ________.
A) molecular formula
B) configuration
C) conformation
D) constitution
E) structure
G) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Draw 4-tert-butyloctane.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the common name of the following compound? 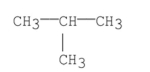
A) isobutane
B) isopropylmethane
C) tert-butane
D) n-butane
E) sec-butane
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has a conformer with two equatorial alkyl substituents?
A) 1,1 -dimethylcyclohexane
B) cis-1,2-dimethylcyclohexane
C) cis-1,3-diethylcyclohexane
D) cis-1,4-diethylcyclohexane
E) trans-1,3-diethylcyclohexane
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many hydrogens are in limonene? 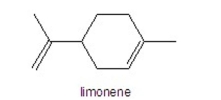
A) 8
B) 10
C) 12
D) 16
E) 18
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the least the least stable conformer of 1-tert-butyl-3- methylcyclohexane.
A) tert-butyl is axial and the methyl is equatorial.
B) tert-butyl is axial and the methyl is axial.
C) tert-butyl is equatorial and the methyl is axial.
D) tert-butyl is equatorial and the methyl is equatorial.
E) All are equally stable.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw all ethers with molecular formula C4H10O.
Correct Answer

verified
Correct Answer
verified
Essay
Draw 4-isopropyl-2-methylheptane.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assuming roughly equivalent molecular weights, which of the following compounds has the highest boiling point?
A) a tertiary amine
B) a quaternary ammonium salt
C) an alcohol
D) an ether
E) an alkyl chloride
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Name the alkane shown below. 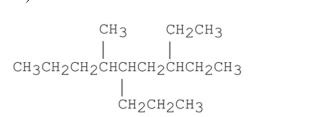
Correct Answer

verified
3-ethyl-6-...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 41 - 60 of 102
Related Exams