A) a compound.
B) an element.
C) a heterogeneous mixture.
D) a homogeneous mixture.
E) a crystalline solid.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What does "X" represent in the following symbol? 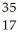 X
X
A) selenium
B) krypton
C) sulfur
D) bromine
E) chlorine
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the mass (in kg) of 4.87 × 1025 atoms of Zn.
A) 5.29 kg
B) 1.89 kg
C) 8.09 kg
D) 1.24 kg
E) 1.09 kg
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All statements about scientific theories are true EXCEPT
A) they explain why nature behaves the way it does.
B) they must have the ability to make predictions on future behavior.
C) they should use observations to test the theory.
D) they are derived from hypothesis.
E) they are speculation.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How are gases different from solids and liquids?
A) Gases can only be made up of atoms.
B) The particles in a gas attract each other much more strongly than in solids and liquids.
C) Gases are compressible.
D) Only gases can take the shape of their container.
E) Gases are colorless.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the characteristics of a liquid.
A) definite volume and definite shape
B) definite volume and no definite shape
C) no definite shape and definite volume
D) no definite shape and no definite volume
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What element is defined by the following information? P+ = 11 n° = 12 e- = 11
A) sodium
B) vanadium
C) magnesium
D) titanium
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the atomic mass of element "X," if it has 2 naturally occurring isotopes with the following masses and natural abundances. X-45 44.8776 amu 32.88% X-47 46.9443 amu 67.12%
A) 46.26 amu
B) 45.91 amu
C) 46.34 amu
D) 46.84 amu
E) 44.99 amu
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the element that has an atomic number of 20.
A) boron
B) neon
C) calcium
D) zinc
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic number for iron is
A) 192.22
B) 77
C) 55.85
D) 26
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the atomic mass of magnesium if magnesium has 3 naturally occurring stable isotopes with the following masses and natural abundances. Mg-24 23.9996 amu 78.99% Mg-25 24.9858 amu 10.00% Mg-26 25.9826 amu 11.01%
A) 22.53 amu
B) 24.32 amu
C) 24.86 amu
D) 25.41 amu
E) 23.92 amu
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the pure substance from the list below.
A) sea water
B) diamond
C) air
D) coffee
E) milk
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom of 17O contains ________ protons.
A) 8
B) 25
C) 9
D) 11
E) 17
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gallium has an atomic mass of 69.723 amu.The Ga-69 (68.926 amu) is 60.111%.What is the amu of the other isotope?
A) 70.924 amu
B) 70.932 amu
C) 70.928 amu
D) 70.920 amu
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about subatomic particles is TRUE?
A) A neutral atom contains the same number of protons and electrons.
B) Protons have about the same mass as electrons.
C) Electrons make up most of the mass of an atom.
D) Protons and neutrons have opposite, but equal in magnitude, charges.
E) Neutrons and electrons are found in the nucleus of an atom.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the characteristics of a solid.
A) definite volume and definite shape
B) definite volume and no definite shape
C) no definite shape and definite volume
D) no definite shape and no definite volume
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the composition of hydrogen peroxide.
A) two hydrogen atoms and two oxygen atoms
B) one hydrogen atom and one oxygen atom
C) two hydrogen atoms and one oxygen atom
D) one hydrogen atom and two oxygen atoms
F) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 121 - 137 of 137
Related Exams