A) -1
B) 0
C) +1
D) +2
E) -2
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules are polar? PCl5 COS XeO3 SeBr2
A) 2
B) 0
C) 1
D) 3
E) 4
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the compound with the smallest percent ionic character.
A) HF
B) IBr
C) HCl
D) LiF
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the number of electron groups around a molecule with a tetrahedral shape.
A) 1
B) 2
C) 3
D) 4
E) 5
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of PF5.
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal
B) eg = octahedral, mg = octahedral
C) eg = trigonal bipyramidal, mg = tetrahedral
D) eg = tetrahedral, mg = trigonal pyramidal
E) eg = trigonal planar, mg = octahedral
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) ,molecular geometry (mg) ,and polarity of SO2.
A) eg = tetrahedral, mg = bent, polar
B) eg = trigonal planar, mg = bent, polar
C) eg = linear, mg = linear, nonpolar
D) eg = tetrahedral, mg = tetrahedral, nonpolar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the bond with the highest bond energy.
A) Si = O
B) N = N
C) C = C
D) C = N
E) O = O
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the strongest bond.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same strength.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of the underlined carbon in CH3CN.
A) eg = tetrahedral, mg = bent
B) eg = linear, mg = bent
C) eg = trigonal planar, mg = tetrahedral
D) eg = linear, mg = linear
E) eg = bent, mg = tetrahedral
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following in order of increasing bond length. C-F C-S C-Cl
A) C-S < C-Cl < C-F
B) C-Cl < C-F < C-S
C) C-F < C-S < C-Cl
D) C-F < C-Cl < C-S
E) C-S < C-F < C-Cl
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Define bond energy.
Correct Answer

verified
Bond energy is the e...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify the compound with the smallest dipole moment in the gas phase.
A) Cl2
B) ClF
C) HF
D) LiF
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) ,molecular geometry (mg) ,and polarity of PCl3.
A) eg = tetrahedral, mg = bent, polar
B) eg = trigonal planar, mg = trigonal planar, nonpolar
C) eg = linear, mg = linear, nonpolar
D) eg = tetrahedral, mg = trigonal pyramidal, polar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following elements in order of increasing electronegativity. Li Fr P
A) P < Li < Fr
B) Li < P < Fr
C) Fr < P < Li
D) Fr < Li < P
E) P < Fr < Li
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following in order of decreasing bond length. H-F H-I H-Br
A) H-F > H-Br > H-I
B) H-I > H-F > H-Br
C) H-I > H-Br > H-F
D) H-Br > H-F > H-I
E) H-F > H-I > H-Br
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bond angle in H2O is
A) 107°
B) 104.5°
C) 120°
D) 109.5°
E) 95°
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many lone pairs of electrons are on the Br atom in BrF4+?
A) 0
B) 1
C) 2
D) 3
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following in order of decreasing dipole moment. I.cis-CHCl = CHCl II.trans-CHCl = CHCI III.cis-CHF = CHF
A) III > I > II
B) II > I > III
C) I > III > II
D) II > III > I
E) I = III > II
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the molecule below.Determine the molecular geometry at each of the 3 labeled atoms. 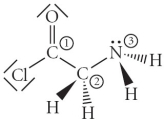
A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the best Lewis structure for NO3⁻.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 138
Related Exams