B) False
Correct Answer

verified
Correct Answer
verified
True/False
The molecule CO2 is linear.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons will chlorine gain or lose when it forms an ion?
A) lose 1
B) gain 1
C) lose 7
D) gain 2
E) lose 3
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The formula for iron(II)sulfide is FeS.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ionic bonding is expected in which of these compounds?
A) ![]()
B) KF
C) ![]()
D) HF
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Matching
Give the correct valence for ions of the following elements.
Correct Answer
Multiple Choice
Double and triple bonds form because ________.
A) the atoms involved have high electronegativities
B) single covalent bonds do not give all of the atoms in the molecule eight valence electrons
C) one of the atoms in the molecule has more than 8 valence electrons
D) the ions involved have charges larger than one
E) there is at least one hydrogen atom involved in the bond
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of the 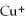 ion is ________.
ion is ________.
A) copper(II)
B) copper(I)
C) copper(III)
D) copper
E) cuprum
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following polyatomic ions has a 3- ionic charge?
A) hydroxide
B) nitrate
C) sulfate
D) phosphate
E) hydrogen carbonate
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to naming rules,the types of compound that use prefixes in their names are ________.
A) ionic compounds
B) ionic compounds involving transition metals
C) polyatomic ions
D) molecular compounds
E) compounds that contain polyatomic ions
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Identify each of the following compounds as covalent or ionic. -dihydrogen sulfide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct formula for the iron(II) ion?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula of carbon tetraiodide?
A) CI
B) ![]()
C) ![]() I
I
D) ![]()
E) ![]()
![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements does not exist as a diatomic molecule?
A) hydrogen
B) nitrogen
C) chlorine
D) oxygen
E) carbon
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In ionic compounds,________ lose their valence electrons to form positively charged ________.
A) metals,anions
B) nonmetals,cations
C) metals,polyatomic ions
D) nonmetals,anions
E) metals,cations
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances contains a nonpolar covalent bond?
A) H2O
B) NaCl
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An anion always ________.
A) has a positive charge
B) contains a group of two or more atoms with a positive charge
C) contains a metal and a nonmetal
D) forms covalent bonds
E) has a negative charge
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds contains an ion with a 3+ charge?
A) KCl
B) ![]() O
O
C) ![]()
D) CuCl
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Matching
Indicate the type of bonding you would expect between the following elements.
Correct Answer
Multiple Choice
The main type of attractive forces between molecules of hydrogen (H2) are ________.
A) ionic bonds
B) hydrogen bonds
C) polar covalent
D) dipole-dipole attractions
E) dispersion forces
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 103
Related Exams