Correct Answer

verified
Correct Answer
verified
Essay
3-Pentanol can be oxidized to 3-pentanone using sodium dichromate. How can the completeness of conversion be gauged using IR spectroscopy?
Correct Answer

verified
The absence of a strong, broad...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which compound would be expected to show intense IR absorption at 3300 cm-1?
A) CH3C꓿CCH3
B) butane
C) but-1-ene
D) CH3CH2C꓿CH
F) None of the above
Correct Answer

verified
D
Correct Answer
verified
Short Answer
Rank the following bonds in order of increasing stretching frequency (cm-1) in IR spectroscopy: 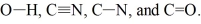
Correct Answer

verified
CN < C=O ...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
How could IR spectroscopy be used to distinguish between the following pair of compounds? CH2=CHCH2CH(CH3)2 and CH3CH2CH2CH(CH3)2
Correct Answer

verified
C-C stretch around 1640 cm-1; vinylic CH stretch above 3000 cm-1
Correct Answer
verified
Short Answer
Deduce a possible structure for the compound with the IR absorptions below. C3H5N: 2950, 2250 cm-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures is consistent with the IR spectra shown below? 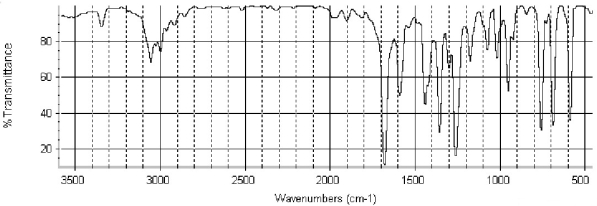
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
In an IR spectrometer, the ________ uses prisms or diffraction gratings to allow only one frequency of light to enter the detector at a time.
Correct Answer

verified
Correct Answer
verified
Short Answer
What wavelength in mm is equivalent to a wavenumber of 1750 cm-1?
Correct Answer

verified
5.71 mm
Correct Answer
verified
Multiple Choice
Which of the following does not have a broad absorption with one or more spikes that is centered about 3300 cm-1 in the IR?
A) (CH3CH2CH2) 3N
B) (CH3CH2CH2) 2NH
C) CH3CH2CH2NH2
D) (CH3) 3CNH2
E) (CH2=CHCH2) 2NH
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide an equation which relates the energy of a photon to its wavelength.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What IR absorption is characteristic of the O-H stretch in alcohols?
A) 4000 cm-1
B) 3300 cm-1
C) 2950 cm-1
D) 2250 cm-1
E) 1710 cm-1
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass spectrum of which compound has M+ and M+2 peaks of approximately equal intensity?
A) 3-bromopentane
B) 3-pentanol
C) pentane
D) 3-chloropentane
E) 3-iodopentane
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Describe the fate of a molecule from introduction to detection in a mass spectrometer.
Correct Answer

verified
Upon introduction, sample molecules are ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Absorption of what type of electromagnetic radiation results in transitions among allowed rotational motions?
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond in the structure below would give rise to the highest absorption frequency in an IR spectrum? 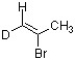
A) sp2 C-H
B) sp3 C-H
C) C-D
D) C=C
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass spectrum of alcohols often fail to exhibit detectable M peaks but instead show relatively large ________ peaks.
A) M+1
B) M+2
C) M-16
D) M-17
E) M-18
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following factors influences how intensely a functional group absorbs infrared radiation (large peak vs. small peak) ?
A) mass of atoms
B) dipole moment
C) bond strength
D) bond length
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures is consistent with the IR spectra shown below? 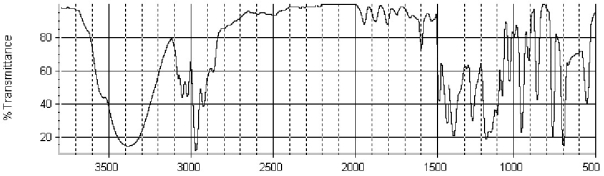
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Absorption of what type of electromagnetic radiation results in transitions among allowed nuclear magnetic spin states?
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 120
Related Exams